Our Success Stories
GPOs chose GS1 GDSN to improve data quality and digitalis processes
The top four healthcare group purchasing organisations (GPOs) in Germany, EKK plus, P.E.G., Prospitalia and Sana Einkauf & Logistik collaborated to create a data sharing platform. Just two years after its launch, more than one million products have been published to it.
A year later, the results speak for themselves:
- 30% more products listed
- 35% increase in quality targets achieved for goods and services that must meet ECLASS standards
- 65% increase in quality targets achieved for procurement
- 560% increase in quality targets achieved for logistics
Our Success Stories
GPOs chose GS1 GDSN to improve data quality and digitalis processes
The top four healthcare group purchasing organisations (GPOs) in Germany, EKK plus, P.E.G., Prospitalia and Sana Einkauf & Logistik collaborated to create a data sharing platform. Just two years after its launch, more than one million products have been published to it.
One year later, the results speak for themselves:
- 30% more products listed
- 35% increase in quality targets achieved for goods and services that must meet ECLASS standards
- 65% increase in quality targets achieved for procurement
- 560% increase in quality targets achieved for logistics
Many challenges
Quality assurance of your product data
With COVIN – the Content Validation Network
COVIN aims to significantly and sustainably increase the quality of the product master data provided by the medical technology companies. In the future, manufacturers should be able to check in advance whether their data meets the defined requirements standard. The regulations COVIN required for this have been published and summarized in a table. The COVIN network is a joint project of AGKAMED GmbH, clinicpartner eG, EK-UNICO GmbH, P.E.G. eG, GDEKK, Prospitalia GmbH and Sana Klinik Einkauf GmbH.
#dontfearCOVIN
Supply of your product data
In HCDP – the Healthcare Content Data Portal
HCDP is the new data pool of the group purchasing organizations (GPOs) P.E.G. eG, Prospitalia GmbH and Sana Klinik Einkauf GmbH.
It forms the basis for secure electronic processes in purchasing and logistics in the healthcare facilities, such as eProcurement and the future electronic invoice. Via HCDP, manufacturers and suppliers continuously and uniformly supply the connected health care facilities with validated data via a standardized process.
Registration of your product data
UDI data for Europe and USA
The Medical Device Regulation MDR obliges the manufacturers of medical devices to store the data about themselves and their products for Europe in the EUDAMED. Medical devices offered in the USA must first be electronically registered in the FDA‘s product database (GUDID)
Even though the EU Commission announced on 30.10.2019 that the operation of the EUDAMED database will not start until May 2025, you should not put the issue off for long. In order to register validated product information electronically, more is necessary than its technical provision. It is key to establish your own processes, organisation and systems to ensure the up-to-dateness and quality of the data in the long term.
Delivery of your product data
Quality assured to the NHS –Trusts
To improve the supply chain, NHS England, the largest purchaser of medical devices in the UK, requires product data from devices to be provided electronically via the Global Data Synchronization Network (GDSN). This is not a regulatory requirement, but it is essential for any equipment company which markets its products in the UK to understand and comply with it.
Sign in of your products
In the Dutch Implant Register
In order to track implants quickly and efficiently, it is legally required that the products are registered in the Dutch Implant Register (LIR).
Distribute your product data
Via GS1 GDSN to your customers
Your customers are increasingly demanding up-to-date, detailed and valid product information. And you need to make your processes more efficient. The GS1 GDSN is the way to deliver this data to many of your national and international customers in a standardized and automated process.
Many challenges
Quality assurance of your product data
With COVIN – the Content Validation Network
COVIN aims to significantly and sustainably increase the quality of the product master data provided by the medical technology companies. In the future, manufacturers should be able to check in advance whether their data meets the defined requirements standard. The regulations COVIN required for this have been published and summarized in a table. The COVIN network is a joint project of AGKAMED GmbH, clinicpartner eG, EK-UNICO GmbH, P.E.G. eG, GDEKK, Prospitalia GmbH and Sana Klinik Einkauf GmbH.
#dontfearCOVIN
Supply of your product data
In HCDP – the Healthcare Content Data Portal
HCDP is the new data pool of the group purchasing organizations (GPOs) P.E.G. eG, Prospitalia GmbH and Sana Klinik Einkauf GmbH.
It forms the basis for secure electronic processes in purchasing and logistics in the healthcare facilities, such as eProcurement and the future electronic invoice. Via HCDP, manufacturers and suppliers continuously and uniformly supply the connected health care facilities with validated data via a standardized process.
Registration of your product data
UDI data for Europe and USA
The Medical Device Regulation MDR obliges the manufacturers of medical devices to store the data about themselves and their products for Europe in the EUDAMED. Medical devices offered in the USA must first be electronically registered in the FDA‘s product database (GUDID)
Even though the EU Commission announced on 30.10.2019 that the operation of the EUDAMED database will not start until May 2025, you should not put the issue off for long. In order to register validated product information electronically, more is necessary than its technical provision. It is key to establish your own processes, organisation and systems to ensure the up-to-dateness and quality of the data in the long term.
Delivery of your product data
Quality assured to the NHS –Trusts
To improve the supply chain, NHS England, the largest purchaser of medical devices in the UK, requires product data from devices to be provided electronically via the Global Data Synchronization Network (GDSN). This is not a regulatory requirement, but it is essential for any equipment company which markets its products in the UK to understand and comply with it.
Sign in of your products
In the Dutch Implant Register
In order to track implants quickly and efficiently, it is legally required that the products are registered in the Dutch Implant Register (LIR).
Distribute your product data
Via GS1 GDSN to your customers
Your customers are increasingly demanding up-to-date, detailed and valid product information. And you need to make your processes more efficient. The GS1 GDSN is the way to deliver this data to many of your national and international customers in a standardized and automated process.

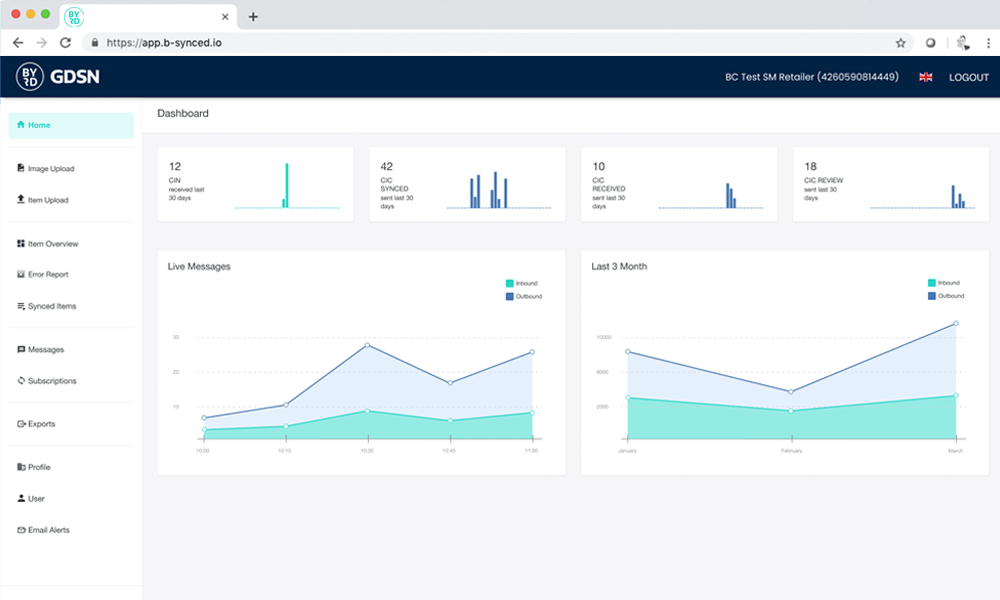
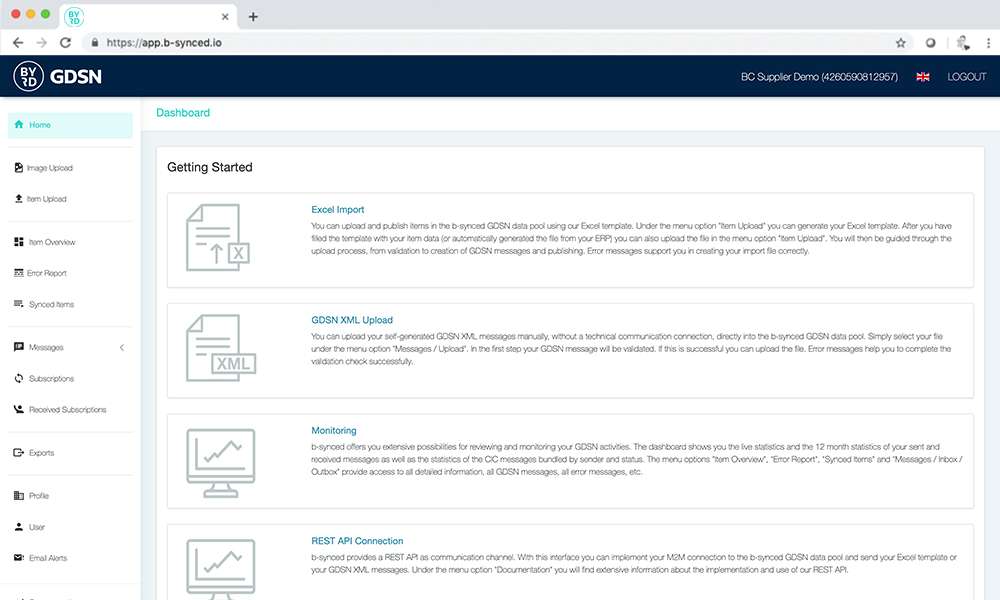
Super easy! And additionally it’s affordable – the Excel upload is already part of your b-synced subscription.
Easy and fast!
The Excel-Upload of b-synced
b-synced provides a simple xlsx template where you can export or manually enter your product data. Simply upload the completed file via the Web UI and b-synced will guide you through the validation and publication process.
If your data recipients perform further validations and inform you of the result via CIC message (CIC = Catalog Item Confirmation), b-synced offers you a sophisticated reporting system that allows you to view the data errors and correct your data.
Super easy! And additionally it’s affordable – the Excel upload is already part of your b-synced subscription.
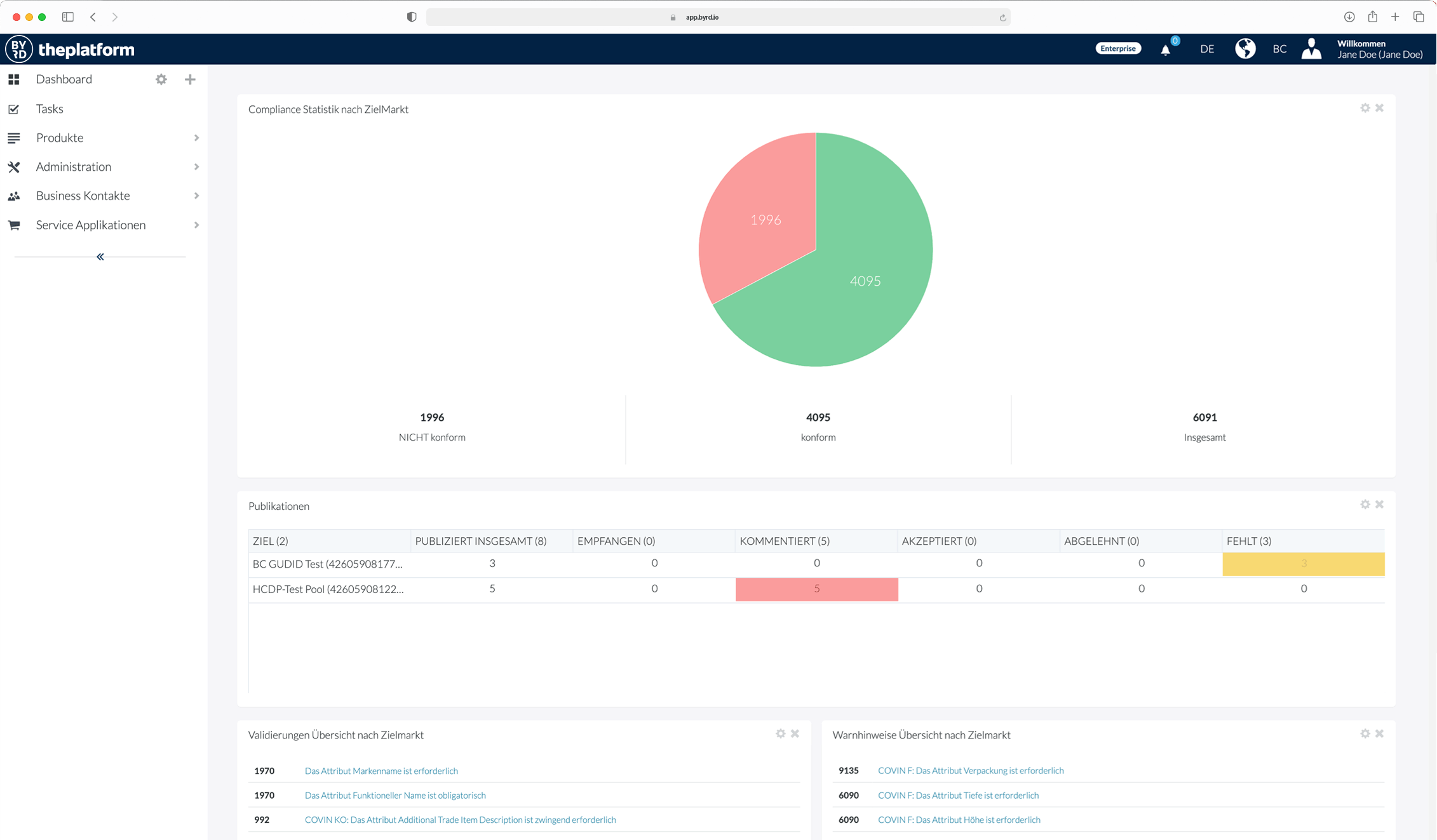
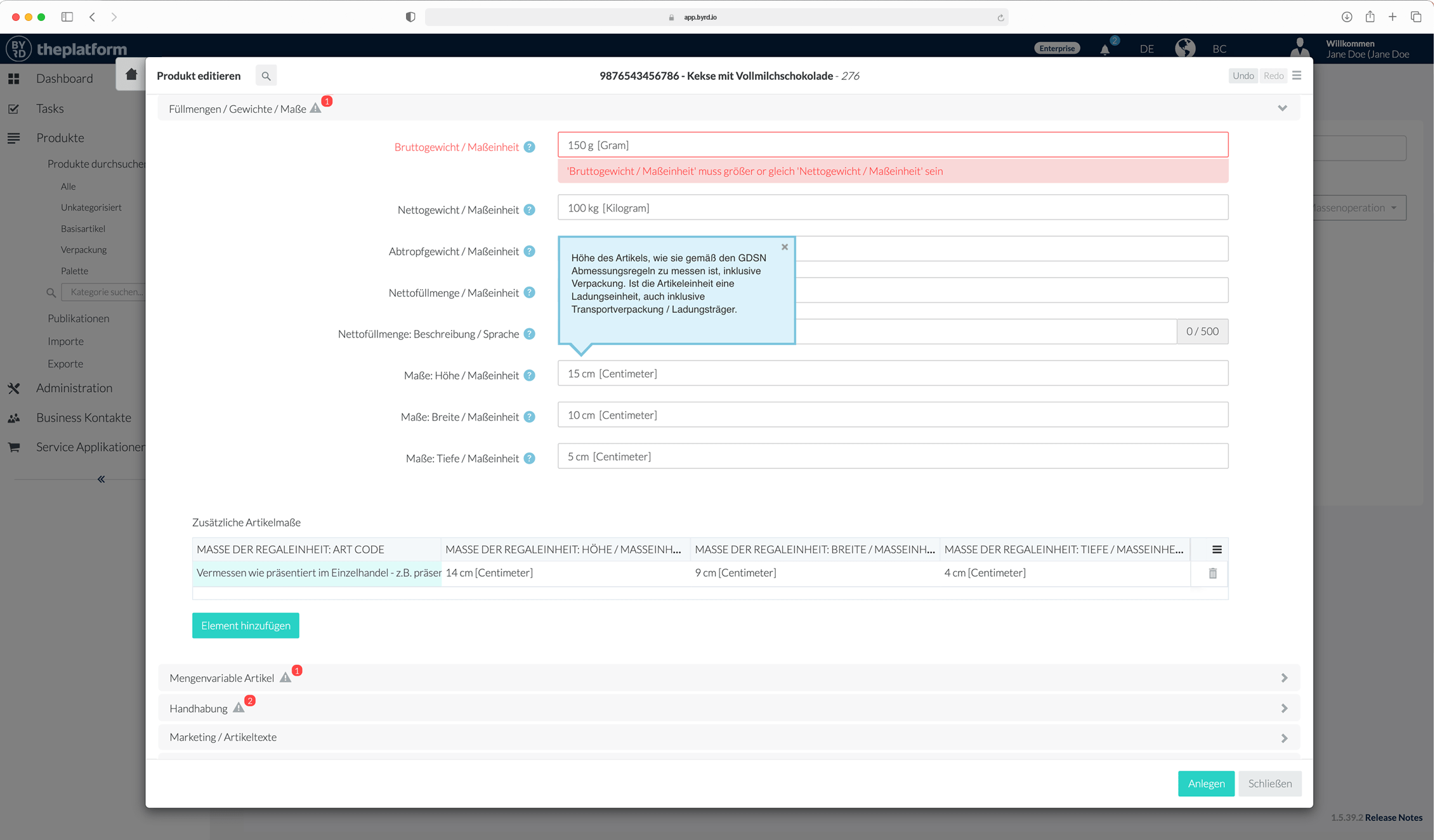
Fast, flexible and automated!
Use of the BYRD Content Syndication Platform
You already have your data available digitally in one or more systems and may only have to add a small part manually – then our cloud PIM and syndication platform BYRD is the solution.
We connect your systems containing product information to BYRD. We set up enrichment and release processes for you, and then your quality-tested product information is already available to your customers.
Fast, flexible and automated!
Use of the BYRD Content Syndication Platform
You already have your data available digitally in one or more systems and may only have to add a small part manually – then our cloud PIM and syndication platform BYRD is the solution.
We connect your systems containing product information to BYRD. We set up enrichment and release processes for you, and then your quality-tested product information is already available to your customers.
The Enterprise Approach!
Implementation of PIM as business process
Do you want to establish Product Information Management as a business process in your company and want to introduce a professional PIM system?
Talk to us!
We support you in the selection and implementation of a professional PIM system. In addition, we ensure a subsequent connection to b-synced and the data supply to your customers via GDSN.
The Enterprise Approach!
Implementation of PIM as a business process
Do you want to establish Product Information Management as a business process in your company and want to introduce a professional PIM system?
Talk to us! We support you in the selection and implementation of a professional PIM system. In addition, we ensure a subsequent connection to b-synced and the data supply to your customers via GDSN.
Selection of our healthcare customers
GS1 GDSN provides high quality product data in a simple and efficient way – view video now!


Manage healthcare data, ensure its quality and share it with your business partners.
Contact us and learn about the benefits of managing healthcare data for your individual business case.